Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Intermolecular Forces
Part b: Types of Intermolecular Forces
Part a:
What are Intermolecular Forces?
Part b: Types of Intermolecular Forces
The Electrical Nature of IMFs
 Intermolecular forces (IMFs) are the forces between different molecules in a sample of a solid or liquid. These forces are electrical in nature. They involve the attraction that exists between positive and negative charges. There can also be repulsive influences between positive charges and between negative charges. We will learn of three types of intermolecular forces - dipole-dipole interactions, hydrogen bonding, and London dispersion forces.
Intermolecular forces (IMFs) are the forces between different molecules in a sample of a solid or liquid. These forces are electrical in nature. They involve the attraction that exists between positive and negative charges. There can also be repulsive influences between positive charges and between negative charges. We will learn of three types of intermolecular forces - dipole-dipole interactions, hydrogen bonding, and London dispersion forces.
Dipole-Dipole Interactions
One type of intermolecular force observed in molecular compounds is the dipole-dipole interaction. These types of forces are only noticeable among polar molecules. As we discussed in Chapter 6, polar molecules have a non-uniform distribution of electrons about the molecule. A combination of widely electronegativity values among bonded atoms and a molecular geometry that doesn’t result in dipole moment cancellation leads to a polar molecule. Polar molecules are characterized as having a dipole. There is a center of positive and negative charge, represented as δ+ and δ-. We sometimes refer to these as a positive pole and a negative pole.
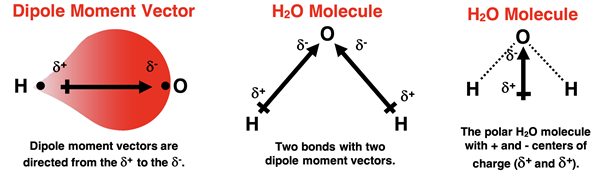
Dipole-dipole interactions occur when the positive pole of one molecule and the negative pole of a neighboring molecule attract each other. Molecules in the liquid and solid state tend to align themselves in such a manner as to maximize the number of attractive forces while minimizing the number of repulsive forces between like-charged poles. Since electrical interactions are strongest at close distance, the + pole of one molecule tries to get as close as possible to the - pole of the neighboring molecule.
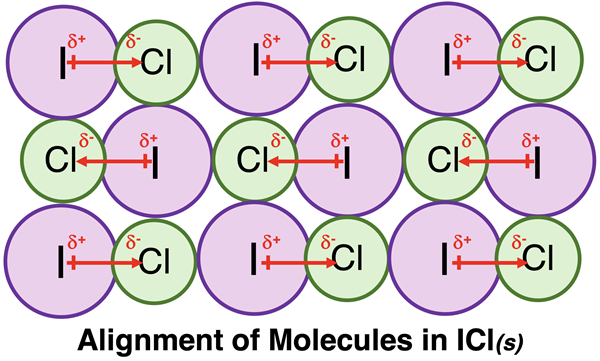
Iodine monochloride, ICl, is (just barely) a solid at room temperature (melting point = 27°C). The I-Cl bond is a polar bond, with the Cl atom being the more electronegative atom. The molecule is polar and has a dipole moment directed from the iodine atom to the Cl atom. The diagram below depicts a typical arrangement of ICl molecules. As can be seen, this arrangement maximizes the attractive force by placing δ+ and δ- poles as close as possible. Like-charged poles are more distant from one another, thus minimizing the repulsions. The overall effect is that the dipole-dipole interactions are strong enough to hold the ICl molecules in the solid state at room temperature.

Chloroform, CHCl3, is a liquid at room temperature. It is a polar molecule with a dipole moment vector. Unlike solid ICl, molecules of CHCl3 are not held in a locked position and have some freedom of movement about the bulk of the liquid. As they move, the molecules tend to momentarily align in given regions of the liquid for short durations of time so as to maximize the attractions between dipoles. These dipole-dipole interactions are of sufficient strength to hold CHCl3 in the liquid state.
Only polar molecules can experience dipole-dipole interactions. The strength of the dipole-dipole interactions increases with increasing molecular polarity. The size of the molecules also matter. Smaller molecules can position themselves closer to one another. Electrical forces of attraction are greatest at close distances. These two attributes leads us to our next type of intermolecular force - hydrogen bonding.
Hydrogen Bonding
The graph below displays boiling point trends for molecular compounds composed of hydrogen and the first four elements of Group 14 (in black) and Group 16 (in red).As we learned on the first page of Lesson 1, the act of boiling is the act of overcoming the intermolecular forces that hold molecules together in the liquid state. Higher boiling points are associated with strong intermolecular forces. An inspection of the data displays a clear trend: as the period number of the element combined with hydrogen in the compound decreases, the boiling point decreases. We would reason that intermolecular forces in hydrogen compounds of Group 14 and 16 elements are strongest when hydrogen is with elements lower in the periodic table. BUT there is one clear exception to this trend. Do you see it?
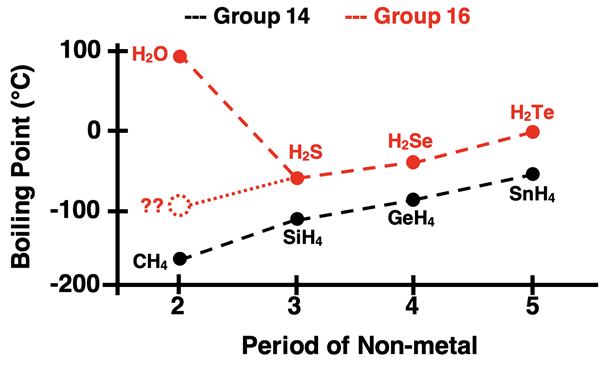
Water, H2O,violates the general trend. It is an exception to the rule. It’s boiling point is significantly higher than what we might expect based on the pattern. We would reason that there is something about H2O that makes the strength of its intermolecular forces stronger than the other Group 16 hydrides. Before we explain, let’s look at two more sets of hydrogen compounds - those of Group 15 and Group 17 elements. The data is displayed below. We see two more trend busters - NH3 and HF.
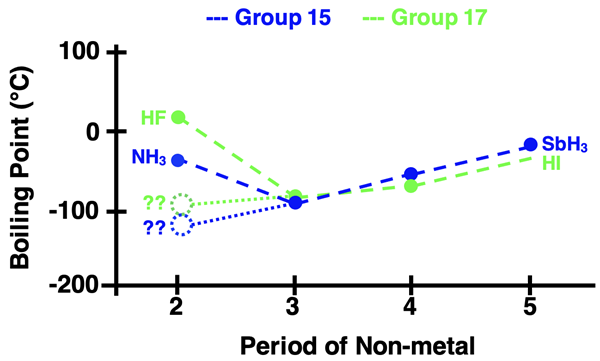
These three boiling point exceptions are one of several pieces of evidence that have led scientists to an understanding of an intermolecular force type known as hydrogen bonding. At its very core, hydrogen bonding is a form of dipole-dipole interaction. But it has earned its unique IMF type because it is exceptionally stronger than all other dipole-dipole interactions. Hydrogen bonding is only observed in molecular compounds that contain a hydrogen atom attached to either an N, O, or F atom having an unshared or lone pair of electrons. Here are the Lewis electron dot structures of the three molecules:
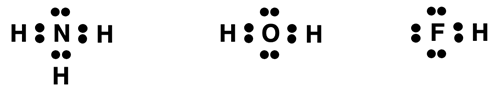
 How does hydrogen bonding work? And what makes this IMF so strong relative to other intermolecular forces? The N-H, O-H, and F-H bonds have two traits that contribute to the strength of hydrogen bonding. First, they are very polar bonds. The elements N, O, and F are the most electronegative elements on the periodic table. When bonded to hydrogen, a bond with a strong dipole moment is formed. Second, these atoms are among the smallest of atoms found on the Periodic Table. When these two traits are combined, the result is a molecule with strongly polar bonds that are small enough to allow for the close approach of dipoles in neighboring molecules. And as we mentioned, electrical forces are greatest at close distances.
How does hydrogen bonding work? And what makes this IMF so strong relative to other intermolecular forces? The N-H, O-H, and F-H bonds have two traits that contribute to the strength of hydrogen bonding. First, they are very polar bonds. The elements N, O, and F are the most electronegative elements on the periodic table. When bonded to hydrogen, a bond with a strong dipole moment is formed. Second, these atoms are among the smallest of atoms found on the Periodic Table. When these two traits are combined, the result is a molecule with strongly polar bonds that are small enough to allow for the close approach of dipoles in neighboring molecules. And as we mentioned, electrical forces are greatest at close distances.

 London Dispersion Forces
London Dispersion Forces
Dipole-dipole interactions and the more specific hydrogen bonding explain the intermolecular forces in polar molecules. But nonpolar molecules will not experience either type of IMF. The third type of intermolecular force - London dispersion forces - is the only force that a nonpolar molecule will experience. The fact is that all molecules experience London dispersion forces; but for nonpolar molecules, it is the only IMF they do experience. How do London dispersion forces work?
To understand London dispersion forces, let’s consider one of the simplest molecules - hydrogen (H2). The two electrons in hydrogen are in constant, random motion. At any given moment in time, both electrons might be temporarily located on the same H atom. This creates a non-uniform electron cloud and a temporary dipole moment within the molecule. During this brief moment in time, this temporary dipole moment might distort the electron clouds of neighboring molecules. This distortion of the electron distribution in a second and perhaps third or fourth nearby molecule will induce a dipole moment in these molecules. These temporarily induced dipoles interact with the original dipole and result in an attractive force. We refer to these attractive forces as London dispersion forces.
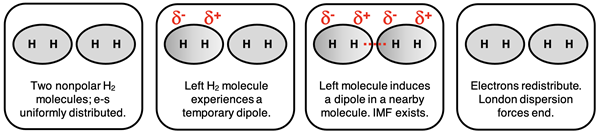
 London dispersions forces tend to be the weakest of the three IMF types. They are very short-lived forces that last for as little as 10-15 seconds. They continuously form and break throughout the bulk of the sample because electrons are in constant, random motion. Temporary dipoles are continuously forming, inducing dipoles in nearby molecules, and leading to short-lived attractive forces before breaking up and forming again at another location.
London dispersions forces tend to be the weakest of the three IMF types. They are very short-lived forces that last for as little as 10-15 seconds. They continuously form and break throughout the bulk of the sample because electrons are in constant, random motion. Temporary dipoles are continuously forming, inducing dipoles in nearby molecules, and leading to short-lived attractive forces before breaking up and forming again at another location.
There tends to be two factors that enhance the strength of London dispersion forces. One of those factors is the size of the molecule. Larger molecules tend to have larger electron clouds that can be more easily distorted. The second factor is the ability of the molecules to align geometrically in such a manner that atoms of nearby molecules are as close to one another as possible. We will discuss each of these factors.
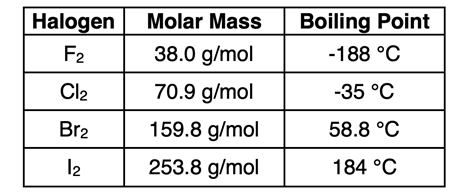
Size Factor: The table at the right shows the boiling points of the four nonmetal, diatomic halogens in periods 2 through 5. You will note that the boiling points increase as you continue down the column. Fluorine and chlorine are both gases at room temperature. Bromine is a liquid at room temperature. And iodine, with a melting point of 114°C, is a solid at room temperature. This data illustrates the effect of size on intermolecular forces. All four halogens are nonpolar molecules. The only IMF that they experience is the London dispersion force. Since boiling points increase with increasing size, it is reasonable to conclude that the strength of the London dispersion forces also increase with increasing size.
 The general strategy for investigating the effect of size upon London dispersion forces is to select a class of nonpolar, molecular compounds that are similar in as many ways as possible except for the size of the molecule. The molar mass is a good indicator of molecule size. Then pick a property like boiling point that provides a good indicator of the strength of the intermolecular forces. This strategy was utilized for the halogen nonmetals above. It is utilized again at the right for a class of compounds known as straight-chain alkanes. These are nonpolar hydrocarbon compounds. We observe again that the boiling points are increasing as the size is increasing. Therefore, London dispersion forces are stronger for the larger molecules.
The general strategy for investigating the effect of size upon London dispersion forces is to select a class of nonpolar, molecular compounds that are similar in as many ways as possible except for the size of the molecule. The molar mass is a good indicator of molecule size. Then pick a property like boiling point that provides a good indicator of the strength of the intermolecular forces. This strategy was utilized for the halogen nonmetals above. It is utilized again at the right for a class of compounds known as straight-chain alkanes. These are nonpolar hydrocarbon compounds. We observe again that the boiling points are increasing as the size is increasing. Therefore, London dispersion forces are stronger for the larger molecules.
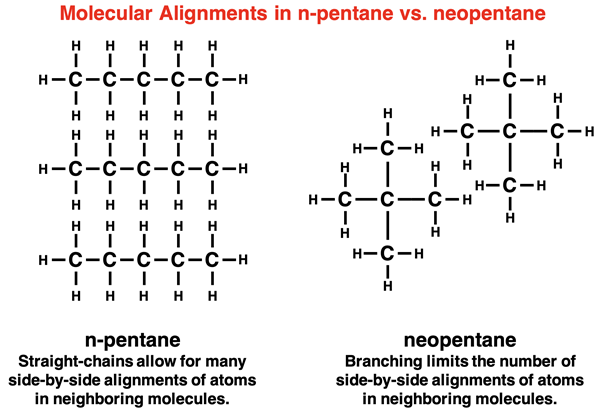
Molecular Alignment Factor: Let’s consider three different non-polar molecules that have the same molecular formula - C5H12 - but a different molecular structure. Their names, condensed formulae, and boiling points are shown below.
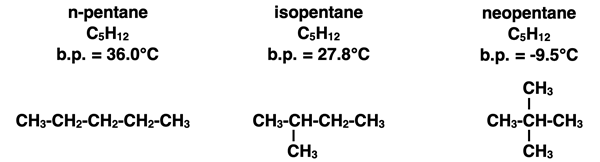
These three compounds are alike in every way with the exception of how their carbon atoms are arranged. All five carbon atoms in n-pentane are arranged as a straight-chain. On the opposite extreme, three carbon atoms in neopentane are lined up in a straight chain with two other carbon atoms branching off the chain. The arrangement of all five carbon atoms in a straight chain (n-pentane) allows one molecule to align with a neighboring molecule in such a manner as to maximize the number of side-by-side atoms. This means that if a temporary dipole develops in one of the bonds, it will more likely be closer to a bond in a neighboring molecule and induce a dipole. This trait maximizes the strength of London dispersion forces and causes the boiling point to be greatest for the straightest chains.
Summary
The three types of intermolecular forces are summarized below. They have been ordered along a spectrum by increasing strength.

These intermolecular forces have a significant influence upon the properties of matter. In Lesson 2, we will investigate how IMFs affect the properties of liquids.
Before You Leave
- Download our Study Card on Intermolecular Force Types. Save it to a safe location and use it as a review tool. (Coming Soon)
- Try our Intermolecular Forces Concept Builder. The activity is a great follow-up to this page.
- Science Reasoning Center activity title Intermolecular Forces. The activity will take you on a journey through how we learn about the relative strength of IMFs.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. For the following pairs of molecules, which would have the strongest dipole-dipole interactions?
- HBr vs. HCl
- CH2F2 vs. CH2Cl2 (The two H atoms and two halogen atoms bond to C.)
- ICl vs. IBr
2. For the following pairs of molecules, which would have the strongest London dispersion forces?
- F2 vs. I2
- AsH3 vs. PH3
- CH4 vs. SiH4
3. Consider the boiling points of the following three noble gases:
He: -272°C
Ar: -186°C
Xe: -107°C
In which case are the interparticle attractions the greatest? And how would you describe these interparticle attractions and explain their relative strength for the three elements?
4. For the following set of molecules, identify which type(s) of intermolecular forces exist. Select from one or more of dipole-dipole interactions, hydrogen bonding, and London dispersion forces
- SO2 (bent structure)
- NH3
- PH3
- SCl2 (bent structure)
- CH4
- HCl
- NF3
- CH2F2 (The two H atoms and two F atoms bonded to C.)
- C3H8
- H3C-O-H (Three H atoms and one O atom bonded to C.)
- H2S
For Questions #5 - #8: Consider the Lewis electron dot diagrams below in answering the following questions.

5. Which of the nine molecules would exhibit hydrogen bonding?
6. Which of the nine molecules would exhibit dipole-dipole interactions but not hydrogen bonding?
7. Which two of the molecules have the same molecular formula (i.e., same number of atoms of each element) despite having different molecular structures?
8. Of the two molecules above, which would have the higher boiling point? Explain the rationale for your answer.